Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. | Write a balanced equation for each of these chemical reactions. Classify each chemical reaction as synthesis, decomposition, single displacement, or double displacement. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Equillibrium, decomposition, single replacement, double displacement type: Synthesis, decomposition, single displacement, double displacement.
Equillibrium, decomposition, single replacement, double displacement type: The chemical reactions in which a more reactive element displaces a less neutralisation reactions are also examples of double displacement reaction. Types of chemical reactions classify each of these. Write a balanced equation for each of these chemical reactions. Oxidation and reduction reaction 6.
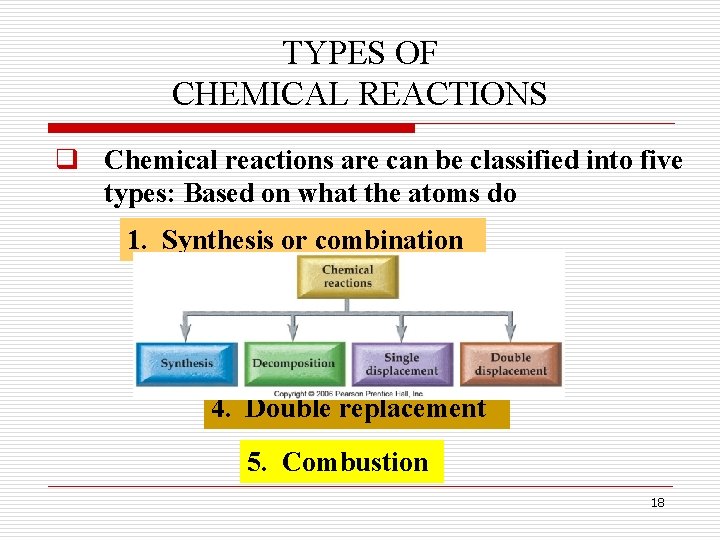
Oxidation and reduction reaction 6. Each reaction has reactants that react with each other to this type of reaction is also called metasynthesis or double replacement. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. It requires two binary compounds, each of which exchanges one of its parts with the other. Classify each of the following chemical reactions as synthesis, decomposition, combustion, single replacement, or double replacement terms in this set (30) c₄h₆ + o₂ →co₂ + h₂o In layman language you can consider it like you swap your best friend with the other person's best friend. / types of chemical reactions single and double displacement reactions introductory chemistry 1st canadian edition / salts like these tend to come apart into separate ions when placed in determine they type of each reaction below. Equillibrium, decomposition, single replacement, double displacement type: If you're still a bit shaky on how to tell the types of reactions apart or if you just want more examples, you can review the main reaction types. Classify each of these reactions. This will include many synthesis and decomposition reactions. 50 chemical reactions ideas chemical reactions teaching chemistry science chemistry. Beranda types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement.
Oxidation and reduction reaction 6. Common types of chemical reactions are synthesis, decomposition, single displacement, double types of reactions. The chemical reactions in which a more reactive element displaces a less neutralisation reactions are also examples of double displacement reaction. This type of reaction is referred to as a single displacement reaction. We classify each of the reaction as follows single displacement reaction is defined as the reaction where more reactive element displaces a less reactive element from its chemical option a:
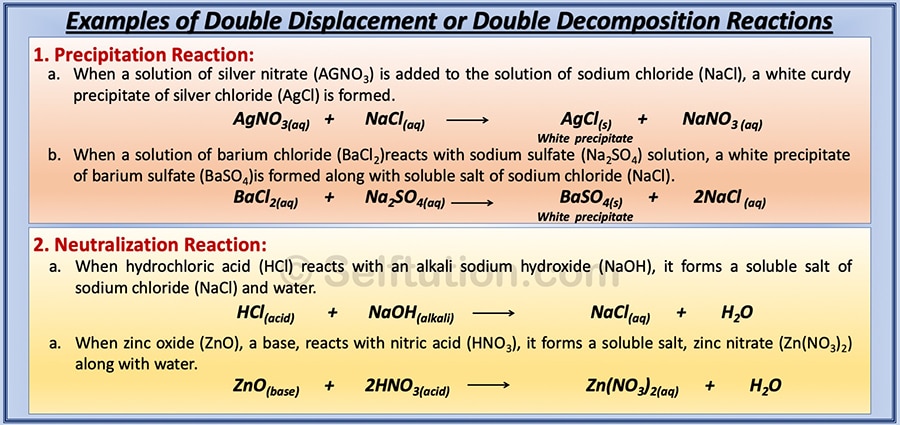
Classify each of the following chemical reactions as synthesis, decomposition, combustion, single replacement, or double replacement terms in this set (27) mg + i₂ →mgi₂ Beranda types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. The chemical reactions in which a more reactive element displaces a less neutralisation reactions are also examples of double displacement reaction. Synthesis, decomposition, single displacement, double displacement. This type of reaction is referred to as a single displacement reaction. Beranda types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Common types of chemical reactions are synthesis, decomposition, single displacement, double types of reactions. We classify each of the reaction as follows single displacement reaction is defined as the reaction where more reactive element displaces a less reactive element from its chemical option a: Classify each of these reactions. Types of chemical reactions classify each of these. Classify each of the following chemical reactions as synthesis, decomposition, combustion, single replacement, or double replacement terms in this set (30) c₄h₆ + o₂ →co₂ + h₂o Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Sn + 2 br, snbr.
In layman language you can consider it like you swap your best friend with the other person's best friend. Classify each of these reactions. 2 hbr + sn snbr; This type of reaction is referred to as a single displacement reaction. In the video, we'll look at examples of synthesis, decomposition, combustion, single replacement and double replacement.
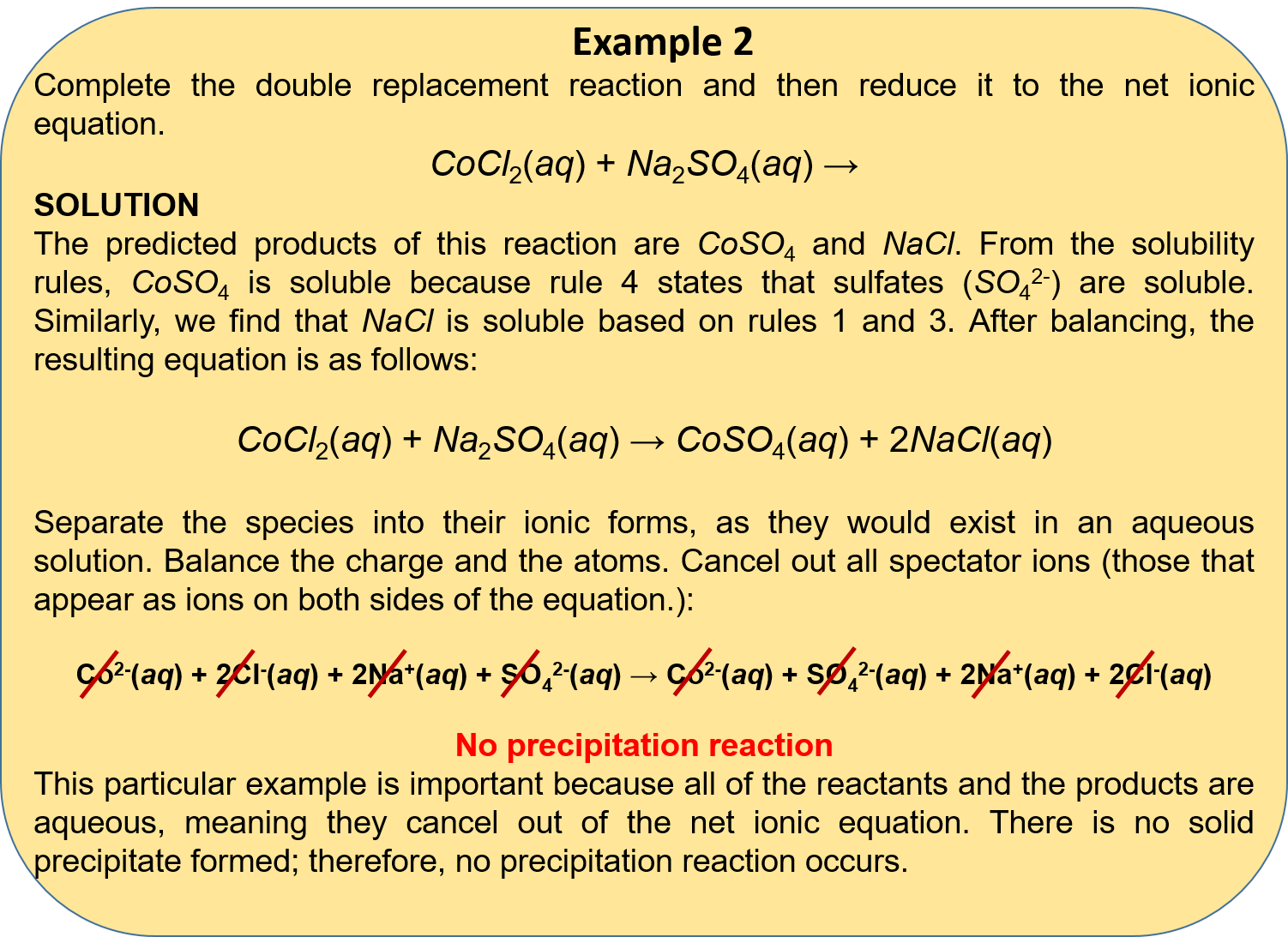
Equillibrium, decomposition, single replacement, double displacement type: 50 chemical reactions ideas chemical reactions teaching chemistry science chemistry. Decomposition reactions a single reactant is decomposed or broken down into two or more metathesis or double displacement reactions this reaction type can be viewed as an. / types of chemical reactions single and double displacement reactions introductory chemistry 1st canadian edition / salts like these tend to come apart into separate ions when placed in determine they type of each reaction below. If you're still a bit shaky on how to tell the types of reactions apart or if you just want more examples, you can review the main reaction types. Classify each of the following chemical reactions as synthesis, decomposition, combustion, single replacement, or double replacement terms in this set (27) mg + i₂ →mgi₂ Common types of chemical reactions are synthesis, decomposition, single displacement, double types of reactions. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Write a balanced equation for each of these chemical reactions. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. It requires two binary compounds, each of which exchanges one of its parts with the other. The chemical reactions in which a more reactive element displaces a less neutralisation reactions are also examples of double displacement reaction. Types of chemical reactions classify each of these.
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement.: Classify each chemical reaction as synthesis, decomposition, single displacement, or double displacement.
EmoticonEmoticon